97.1% Sensitivity Hev Igm Elisa Test Kit for Virus DNA and Rna
Shanghai Eugene Bio tech Co.,Ltd.(Eugene) was established in 2007,which is a subsidiary of CIRC (China Isotope&Radiation
Send your inquiryDESCRIPTION
Basic Info
| Model NO. | CA125 |
| Transport Package | Cartons with Ice Bag |
| Specification | 63*41*37cm |
| Trademark | EUGENE |
| Origin | China |
| Production Capacity | 100, 000/Week |
Product Description

Shanghai Eugene Bio tech.(Eugene) was established in 2007,which is a subsidiary of CIRC (China Isotope&Radiation Corporation, stock code:01763.hk, dedicated to the research and production of colloidal gold, CLIA, ELISA, RIA and other rapid detection methods. Our headquarters is Beijing North Institute of Biological Technology (BNIBT), it was established on July01,1985, which has engaged in researching, producing and selling of in vitro diagnose kits. It's one of the biggest and earliest institutes of the field in China. EUGENE series of human colloidal gold rapid detection products cover infectious diseases such as hepatitis, gastrointestinal, respiratory infections, tropical diseases, sexual transmitted diseases, fertility hormones and natal-healthcare, the rapid detection of tumor markers, cardiovascular disease markers, drugs and alcohol, and so on.Eugene products are widely used in domestic and foreign hospitals, clinics, physical examination centers, disease prevention and control systems, epidemic prevention stations, blood stations, family, laboratories, research institutions, public security and traffic system, etc. We have exported to more than 50 countries in the world, won the consistent praise of the majority of users with excellent performance and excellent service.We Eugene mainly contribution to the human health.As we had supported the Dengue fever epidemic of Bangladesh in 2019.Our Cox-19 test had been registered in Italy,Indonesia,and exported to many countries of the Global market, and receive the good reputation and good feedback by all of our customers.
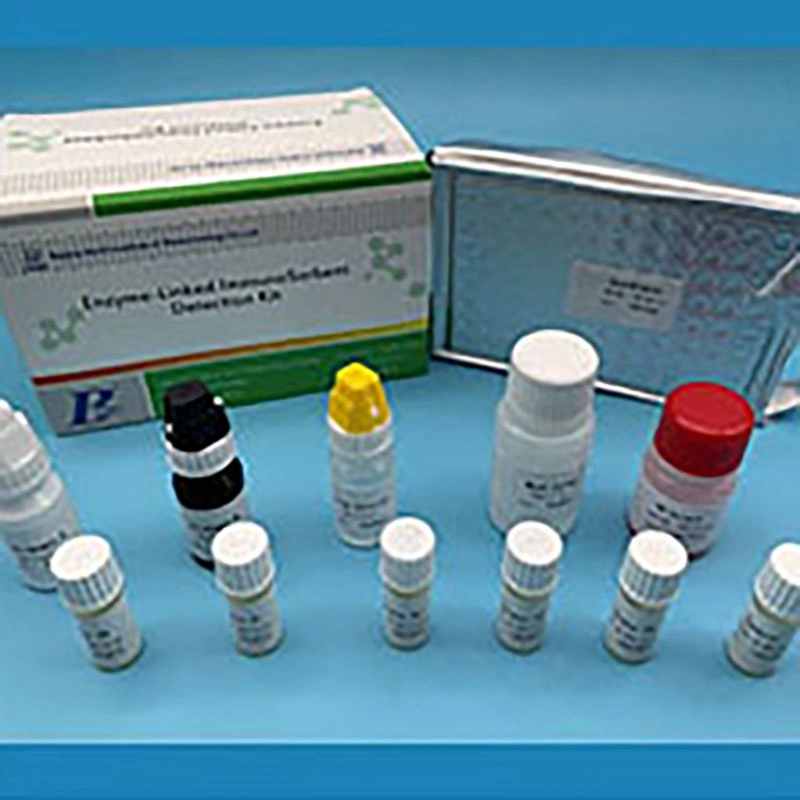
CA-125 uses a "sandwich principle", Enzyme-linked immunological sorbent assay. To measure CA-125 levels in serum, plastic wells coated with a monoclonal antibodies of CA-125 are supplied in the kit. After the patient's specimen and another mono-antibody labelled with HRP are added, CA-125, if present, is fixed to the solid phase antibody and creating a HRP-antibody~ CA-125~antibody "sandwich". After TMB substrate added, the result is obtained by EIA plate reader.1.Coated Microplate: 1 plate (8×12 wells), Ready to use. Coated with anti-CA125 antibody and sealed in an aluminum bag. Remove the strips in the resealable bag with a desiccant to protect from moisture after opened. Store at 2-8ºC until expiration date.2.HRP Conjugate: 1 vial of 6ml, Ready to use. Containing HRP labeled anti-CA125 and proclin-300 preservative. Store at 2-8ºC until expiration date.3.Calibrator: 4 vials of 0.5ml, Ready to use. Labeled with S1 to S4 and the concentration of CA125 is 15, 40, 100, 250 U/ml. Store at 2-8ºC until expiration date.4.Control: 1 vial of 0.5ml, Ready to use. Store at 2-8ºC until expiration date. The concentration is 57~107 U/ml. 5.Chromogen A: 1 vial of 7ml, Ready to use. Containing H2O2. Store at 2-8ºC until expiration date.6.Chromogen B: 1 vial of 7ml, Ready to use. Containing TMB. Store at 2-8ºC until expiration date. 7.Stop Solution: 1 vial of 7ml, Ready to use. Containing H2SO4. Store at 2-8ºC until expiration date.8.Wash buffer: 1 vial of 15ml, Concentrate 20-fold, diluting with deionized water before the assay. Containing NaCl, phosphate and tween-20. Store at 2-8ºC until expiration date.9.Plate sealer: 2 pieces. 10.Plastic resealable bag with desiccant: 1 set. 11.Instruction manual: 1 copy.